The Smarter Way to Scale: ISV Solutions Over Custom ERP Builds
- Dec 26, 2025
- 2 min read
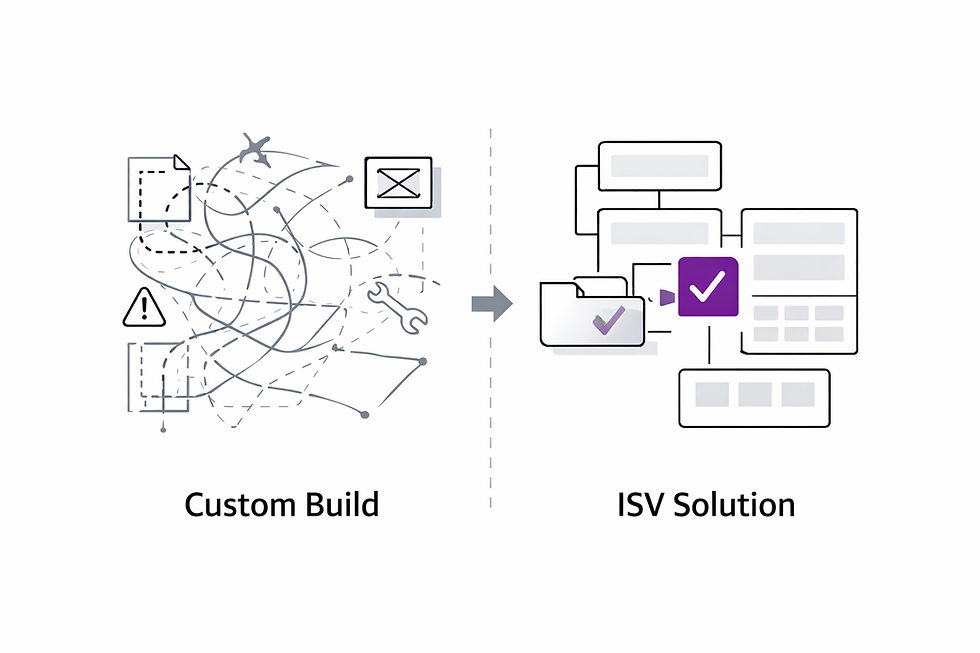
Why ISV Solutions Beat “Build from Scratch” in Life Science
Rolling out Microsoft Dynamics 365 Finance & Supply Chain in a regulated industry is never “just another IT project.” You’re not only mapping business processes — you’re mapping compliance, validation, and quality control into every screen and workflow.
For many life science companies, the temptation is to build every feature themselves. The result? Months of development, endless testing, and custom code that may not survive the next Microsoft update.
That’s where Independent Software Vendor (ISV) solutions come in. Purpose-built for Dynamics 365, they:
Extend core capabilities without reinventing the wheel
Deliver specialized functionality for industry-specific needs
Deploy faster and with less risk than custom builds
Stay compatible with Microsoft’s release cycle, so your upgrades don’t break your processes
Scalability: The Difference Between Surviving and Thriving
Scalability isn’t just about handling more users or bigger data sets — it’s about adapting to change without starting over.
With scalable ISV solutions inside Dynamics 365, you can:
Reuse and replicate proven integrations instead of coding from scratch every time
Stay update-ready because solutions are tested and adjusted before Microsoft pushes a release
Add capabilities quickly — whether it’s EDI, PLM integration, or master data management — without derailing ongoing operations
Keep IT and compliance teams aligned by using solutions built with security, validation, and audit readiness baked in
For example, a life sciences manufacturer integrating a new PLM system can use a prebuilt ISV connector to go live in weeks, not months — without risking data integrity or slowing production.
What to Look for in an ISV Partner
When your ERP is the backbone of regulated operations, your ISV partner needs more than just “compatible code.” Look for:
Industry Experience – They should understand your compliance obligations (GMP, FDA 21 CFR Part 11, ISO standards) and how those shape ERP workflows.
Embedded in Dynamics 365 – The solution should run on the same codebase and user experience as the rest of your ERP.
Update Commitment – They should certify compatibility before each Microsoft update, so you’re never left scrambling.
Low-Code / No-Code Options – So your internal team can own and adapt the solution without constant developer involvement.
Proven ROI – Look for documented case studies showing time-to-value, cost savings, and operational impact.
The Bottom Line
Future-proofing your Dynamics 365 ERP in life sciences isn’t about locking down today’s workflows — it’s about making sure you can scale, adapt, and stay compliant without costly rebuilds.
Maggnumite for Life Science partners with proven ISV providers to deliver:
Faster deployment timelines
Lower total cost of ownership
Built-in compliance features
Guaranteed compatibility with Microsoft updates
Because in regulated manufacturing, every change — whether it’s an ERP upgrade or a new business requirement — needs to be an opportunity, not a disruption.
📅 Book a Demo by contacting info@maggnumite.com to see how scalable ISV solutions can keep your Dynamics 365 environment ready for whatever’s next.


